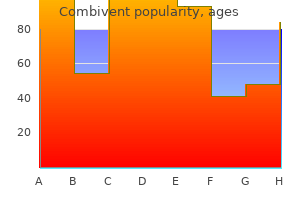
Combivent
Eloise J. Prijoles, M.D.
- Greenwood Genetic Center
- Columbia, South Carolina
Strengthen research cold medications buy generic combivent 100mcg on-line, training and development capabilities mueller sports medicine cheap 100mcg combivent mastercard, particularly in developing countries medicine nobel prize 2016 order cheap combivent online, to support the activities outlined in this programme area; b medications gerd order combivent with paypal. Develop mechanisms for scaling up and disseminating environmentally sound biotechnologies of high environmental importance treatment brown recluse bite buy combivent 100 mcg with amex, especially in the short term treatment zoster ophthalmicus buy combivent 100mcg line, even though those biotechnologies may have limited commercial potential; c. Enhance cooperation, including transfer of biotechnology, between participating countries for capacity-building; d. Develop appropriate safety procedures based on programme area D, taking account of ethical considerations. The Conference secretariat has estimated the average total annual cost (1993-2000) of implementing the activities of this programme to be about $1 billion, including about $10 million from the international community on grant or concessional terms. The activities for this programme area will increase the demand for trained personnel. Support for existing training programmes needs to be increased, for example, at the university and technical institute level, as well as the exchange of trained personnel between countries and regions. New and additional training programmes also need to be developed, for example, for technical and support personnel. There is also an urgent need to improve the level of understanding of biological principles and their policy implications among decision makers in Governments, and financial and other institutions. Relevant institutions will need to have the responsibility for undertaking, and the capacity (political, financial and workforce) to undertake, the above-mentioned activities and to be dynamic in response to new biotechnological developments (see programme area E). Enhancing safety and developing international mechanisms for cooperation Basis for action 16. There is a need for further development of internationally agreed principles on risk assessment and management of all aspects of biotechnology, which should build upon those developed at the national level. Only when adequate and transparent safety and border-control procedures are in place will the community at large be able to derive maximum benefit from, and be in a much better position to accept the potential benefits and risks of, biotechnology. Several fundamental principles could underlie many of these safety procedures, including primary consideration of the organism, building on the principle of familiarity, applied in a flexible framework, taking into account national requirements and recognizing that the logical progression is to start with a step -by-step and case-by case approach, but also recognizing that experience has shown that in many instances a more comprehensive approach should be used, based on the experiences of the first period, leading, inter alia, to streamlining and categorizing; complementary consideration of risk assessment and risk management; and classification into contained use or release to the environment. The aim of this programme area is to ensure safety in biotechnology development, application, exchange and transfer through international agreement on principles to be applied on risk assessment and management, with particular reference to health and environmental considerations, including the widest possible public participation and taking account of ethical considerations. The proposed activities for this programme area call for close international cooperation. They should build upon planned or existing activities to accelerate the environmentally sound application of biotechnology, especially in developing countries. Governments at the appropriate level, with the support of relevant international and regional organizations, the private sector, non-governmental organizations and academic and scientific institutions, should: a. Make the existing safety procedures widely available by collecting the existing information and adapting it to the specific needs of different countries and regions; b. Further develop, as necessary, the existing safety procedures to promote scientific development and categorization in the areas of risk assessment and risk management (information requirements; databases; procedures for assessing risks and conditions of release; establishment of safety conditions; monitoring and inspections, taking account of ongoing national, regional and international initiatives and avoiding duplication wherever possible); c. Compile, update and develop compatible safety procedures into a framework of internationally agreed principles as a basis for guidelines to be applied on safety in biotechnology, including consideration of the need for and feasibility of an international agreement, and promote information exchange as a basis for further development, drawing on the work already undertaken by international or other expert bodies; d. Undertake training programmes at the national and regional levels on the application of the proposed technical guidelines;. Assist in exchanging information about the procedures required for safe handling and risk management and about the conditions of release of the products of biotechnology, and cooperate in providing immediate assistance in cases of emergencies that may arise in conjunction with the use of biotechnology products. Governments at the appropriate level, with the support of the relevant international and regional organizations, should raise awareness of the relative benefits and risks of biotechnology. Organizing one or more regional meetings between countries to identify further practical steps to facilitate international cooperation in bio-safety; b. Establishing an international network incorporating national, regional and global contact points; c. Providing direct assistance upon request through the international network, using information networks, databases and information procedures; d. Considering the need for and feasibility of internationally agreed guidelines on safety in biotechnology releases, including risk assessment and risk management, and considering studying the feasibility of guidelines which could facilitate national legislation on liability and compensation. Actual costs and financial terms, including any that are non concessional, will depend upon, inter alia, the specific strategies and programmes Governments decide upon for implementation. Adequate international technical and financial assistance should be provided and technical cooperation to developing countries facilitated in order to build up technical, managerial, planning and administrative capacities at the national level to support the activities in this programme area (see also programme area E). Establishing enabling mechanisms for the development and the environmentally sound application of biotechnology Basis for action 16. The accelerated development and application of biotechnologies, particularly in developing countries, will require a major effort to build up institutional capacities at the national and regional levels. In developing countries, enabling factors such as training capacity, know-how, research and development facilities and funds, industrial building capacity, capital (including venture capital) protection of intellectual property rights, and expertise in areas including marketing research, technology assessment, socio-economic assessment and safety assessment are frequently inadequate. Efforts will therefore need to be made to build up capacities in these and other areas and to match such efforts with appropriate levels of financial support. There is therefore a need to strengthen the endogenous capacities of developing countries by means of new international initiatives to support research in order to speed up the development and application of both new and conventional biotechnologies to serve the needs of sustainable development at the local, national and regional levels. National mechanisms to allow for informed comment by the public with regard to biotechnology research and application should be part of the process. Some activities at the national, regional and global levels already address the issues outlined in programme areas A, B, C and D, as well as the provisioin of advice to individual countries on the development of national guidelines and systems for the implementation of those guidelines. These activities are generally uncoordinated, however, involving many different organizations, priorities, constituencies, time-scales, funding sources and resource constraints. There is a need for a much more cohesive and coordinated approach to harness available resources in the most effective manner. As with most new technologies, research in biotechnology and the application of its findings could have significant positive and negative socio-economic as well as cultural impacts. These impacts should be carefully identified in the earliest phases of the development of biotechnology in order to enable appropriate management of the consequences of transferring biotechnology. To promote the development and application of biotechnologies, with special emphasis on developing countries, by: i. Providing the necessary support for biotechnology, particularly research and product development, at the national, regional and international levels; iii. Raising public awareness regarding the relative beneficial aspects of and risks related to biotechnology, to contribute to sustainable development; iv. Helping to create a favourable climate for investments, industrial capacity building and distribution/marketing; v. Encouraging the exchange of scientists among all countries and discouraging the "brain drain"; vi. Recognizing and fostering the traditional methods and knowledge of indigenous peoples and their communities and ensuring the opportunity for their participation in the economic and commercial benefits arising from developments in biotechnology; 9/ b. To identify ways and means of enhancing current efforts, building wherever possible on existing enabling mechanisms, particularly regional, to determine the precise nature of the needs for additional initiatives, particularly in respect of developing countries, and to develop appropriate response strategies, including proposals for any new international mechanisms; c. To establish or adapt appropriate mechanisms for safety appraisal and risk assessment at the local, regional and international levels, as appropriate. Governments at the appropriate level, with the support of international and regional organizations, the private sector, non-governmental organizations and academic and scientific institutions, should: a. Develop policies and mobilize additional resources to facilitate greater access to the new biotechnologies, particularly by and among developing countries; b. Implement programmes to create greater awareness of the potential and relative benefits and risks of the environmentally sound application of biotechnology among the public and key decision makers; c. Undertake an urgent review of existing enabling mechanisms, programmes and activities at the national, regional and global levels to identify strengths, weaknesses and gaps, and to assess the priority needs of developing countries; d. Undertake an urgent follow-up and critical review to identify ways and means of strengthening endogenous capacities within and among developing countries for the environmentally sound application of biot echnology, including, as a first step, ways to improve existing mechanisms, particularly at the regional level, and, as a subsequent step, the consideration of possible new international mechanisms, such as regional biotechnology centres;. Develop strategic plans for overcoming targeted constraints by means of appropriate research, product development and marketing; f. Establish additional quality-assurance standards for biotechnology applications and products, where necessary. The following activities should be undertaken: facilitation of access to existing information dissemination systems, especially among developing countries; improvement of such access where appropriate; and consideration of the development of a directory of information. Governments at the appropriate level, with the assistance of international and regional organizations, should develop appropriate new initiatives to identify priority areas for research based on specific problems and facilitate access to new biotechnologies, particularly by and among developing countries, among relevant undertakings within those countries, in order to strengthen endogenous capacities and to support the building of research and institutional capacity in those countries. Workshops, symposia, seminars and other exchanges among the scientific community at the regional and global levels, on specific priority themes, will need to be organized, making full use of the existing scientific and technological manpower in each country for bringing about such exchanges. Personnel development needs will need to be identified and additional training programmes developed at the national, regional and global levels, especially in developing countries. These should be supported by increased training at all levels, graduate, postgraduate and post-doctoral, as well as by the training of technicians and support staff, with particular reference to the generation of trained manpower in consultant services, design, engineering and marketing research. Training programmes for lecturers training scientists and technologists in advanced research institutions in different countries throughout the world will also need to be developed, and systems giving appropriate rewards, incentives and recognition to scientists and technologists will need to be instituted (see para. Conditions of service will also need to be improved at the national level in developing countries to encourage and nurture trained manpower with a view to retaining that manpower locally. Society should be informed of the social and cultural impact of the development and application of biotechnology. Biotechnology research and development is undertaken both under highly sophisticated conditions and at the practical level in many countries. Efforts will be needed to ensure that the necessary infrastructure facilities for research, extension and technology activities are available on a decentralized basis. Global and regional collaboration for basic and applied research and development will also need to be further enhanced and every effort should be made to ensure that existing national and regional facilities are fully utilized. Such institutions already exist in some countries and it should be possible to make use of them for training purposes and joint research projects. Strengthening of universities, technical schools and local research institutions for the development of biotechnologies and extension services for their application will need to be developed, especially in developing countries. The marine environment including the oceans and all seas and adjacent coastal areas forms an integrated whole that is an essential component of the global life-support system and a positive asset that presents opportunities for sustainable development. International law, as reflected in the provisions of the United Nations Convention on the Law of the Sea 1/, 2/ referred to in this chapter of Agenda 21, sets forth rights and obligations of States and provides the international basis upon which to pursue the protection and sustainable development of the marine and coastal environment and its resources. This requires new approaches to marine and coastal area management and development, at the national, subregional, regional and global levels, approaches that are integrated in content and are precautionary and anticipatory in ambit, as reflected in the following programme areas: 3/ a. Integrated management and sustainable development of coastal areas, including exclusive economic zones; b. Sustainable use and conservation of marine living resources under national jurisdiction;. Addressing critical uncertainties for the management of the marine environment and climate change; f. The implementation by developing countries of the activities set forth below shall be commensurate with their individual technological and financial capacities and priorities in allocating resources for development needs and ultimately depends on the technology transfer and financial resources required and made available to them. Integrated management and sustainable development of coastal and marine areas, including exclusive economic zones Basis for action 17. The coastal area contains diverse and productive habitats important for human settlements, development and local subsistence. For small island States or countries, these are the areas most available for development activities. Despite national, subregional, regional and global efforts, current approaches to the management of marine and coastal resources have not always proved capable of achieving sustainable development, and coastal resources and the coastal environment are being rapidly degraded and eroded in many parts of the world. Coastal States commit themselves to integrated management and sustainable development of coastal areas and the marine environment under their national jurisdiction. Provide for an integrated policy and decision-making process, including all involved sectors, to promote compatibility and a balance of uses; b. Apply preventive and precautionary approaches in project planning and implementation, including prior assessment and systematic observation of the impacts of major projects;. Promote the development and application of methods, such as national resource and environmental accounting, that reflect changes in value resulting from uses of coastal and marine areas, including pollution, marine erosion, loss of resources and habitat destruction; f. Provide access, as far as possible, for concerned individuals, groups and organizations to relevant information and opportunities for consultation and participation in planning and decision-making at appropriate levels. Each coastal State should consider establishing, or where necessary strengthening, appropriate coordinating mechanisms (such as a high-level policy planning body) for integrated management and sustainable development of coastal and marine areas and their resources, at both the local and national levels. Such mechanisms should include consultation, as appropriate, with the academic and private sectors, non-governmental organizations, local communities, resource user groups, and indigenous people. Implementation of integrated coastal and marine management and sustainable development plans and programmes at appropriate levels; c. Preparation of coastal profiles identifying critical areas, including eroded zones, physical processes, development patterns, user conflicts and specific priorities for management; d. Prior environmental impact assessment, systematic observation and follow-up of major projects, including the systematic incorporation of results in decision-making;. Contingency plans for human induced and natural disasters, including likely effects of potential climate change and sealevel rise, as well as contingency plans for degradation and pollution of anthropogenic origin, including spills of oil and other materials; f. Improvement of coastal human settlements, especially in housing, drinking water and treatment and disposal of sewage, solid wastes and industrial effluents; g. Periodic assessment of the impacts of external factors and phenomena to ensure that the objectives of integrated management and sustainable development of coastal areas and the marine environment are met; h. Integration of sectoral programmes on sustainable development for settlements, agriculture, tourism, fishing, ports and industries affecting the coastal area; j. Coastal States, with the support of international organizations, upon request, should undertake measures to maintain biological diversity and productivity of marine species and habitats under national jurisdiction. Inter alia, these measures might include: surveys of marine biodiversity, inventories of endangered species and critical coastal and marine habitats; establishment and management of protected areas; and support of scientific research and dissemination of its results. Coastal States, where necessary, should improve their capacity to collect, analyse, assess and use information for sustainable use of resources, including environmental impacts of activities affecting the coastal and marine areas. Information for management purposes should receive priority support in view of the intensity and magnitude of the changes occurring in the coastal and marine areas. Develop and maintain databases for assessment and management of coastal areas and all seas and their resources; b. Conduct regular environmental assessment of the state of the environment of coastal and marine areas; d. Prepare and maintain profiles of coastal area resources, activities, uses, habitats and protected areas based on the criteria of sustainable development;. Cooperation with developing countries, and, where applicable, subregional and regional mechanisms, should be strengthened to improve their capacities to achieve the above.
Terefore symptoms of high blood pressure generic 100 mcg combivent with amex, the main components of insur nifcant potential to reduce the burden of disease and ance schemes are collecting revenue medications rights order discount combivent on-line, pooling resources poverty medicine vicodin order generic combivent canada. Using their power as large-scale purchasers medicine research purchase combivent 100mcg online, and risks medications jejunostomy tube purchase line combivent, and purchasing quality goods and services symptoms genital herpes discount combivent 100 mcg amex. Health insurance is appealing to gov Furthermore, carefully designed insurance-based fnanc ernments because it takes the entire fnancial burden ing for medicines is both scalable and sustainable. However, when governments consider hazard (which in this case refers to more frequent use instituting health insurance, they need to be aware of the of services or medicines by members of an insurance realities of implementation; the complexity of the issues scheme than would occur were they not insured), well involved is ofen poorly understood. Public and private insur a resource-poor setting is still a matter of considerable ance programs control pharmaceutical expenditures debate. Whatever type of health fnancing mechanism a through measures related to payment, management, country decides to adopt, the transition to universal cov prescribing patterns, dispensing practices, and use. In addition to those direct costs, income is health coverage lost when family members are sick, and this loss reinforces the poverty-illness cycle. Women are especially vulnerable, In many countries, especially those with the fewest because they are usually the main family caregivers. Traditionally, governments have pro is fnanced out-of-pocket, a system that places the larg vided a national health service for all citizens with fnanc est burden on the poorest people. Many of these health systems have spending accounts for more than 60 percent of total health not worked well or consistently provided needed medicines, spending in low-income countries (Gottret and Schieber a gap that has led to increased out-of-pocket spending for 2006), of which 60 to 90 percent may be spent on medicines health care services and medicines in the private sector, 12 / Pharmaceutical benefts in insurance programs 12. Evidence from mul dictable, infrequent, costly, unwanted, and uncontrollable tiple countries has shown that a lack of health insurance is by the insured. A good example is insuring a house against a key condition related to catastrophic household spending fre. Many people are prepared to pay a fnancing systems more equitable and increase coverage to regular premium for a lifetime to gain peace of mind against the entire population, many low and middle-income coun a catastrophe that they hope will never happen. Health insurance coverage that includes pharma the risk (for example, pregnancy); and the presence of insur ceuticals has expanded access to medicines in many coun ance increases the use of services. Health insurance schemes appeal to both citizens and The principal aims of well-managed insurance schemes their governments because they help manage the fnancial are to reduce catastrophic fnancial loss in the event of a burden by spreading the total cost of insured health care serious illness and to guarantee the funds or access needed among various partners. In addition, donors and inter to secure necessary, if expensive, medical services. Health national fnancial institutions, such as the World Bank, insurance provides this fnancial protection by evening out are fnding health insurance to be an increasingly feasible household health expenditures. The chapter describes the healthy people subsidize those who consume more sys main components of health insurance and potential prob tem resources and are relatively sick (risk pooling). Health insurance is a mechanism for spreading the risks of Social or public health insurance: The most typical under potential health care costs over a group of individuals or standing of social health insurance is that membership households, with the goal of protecting the individual from is compulsory for a designated population; fnancial a catastrophic fnancial loss in the event of serious illness. In multiple Germany launched its program in 1883 (Carrin and payer systems, several diferent organizations perform all James 2005). Teir insur Private health insurance: Private indemnity insurance ance pools have diferent levels of risk, and consumers may is (usually) paid for by voluntary contributions from be able to choose their own insurer. In high-income countries, private health insurance ing and achieving fnancial sustainability for pharmaceuti either replaces or supplements public coverage. Netherlands has the greatest proportion of population covered by some sort of private health insurance, while Revenue collection the United States and Uruguay are countries with the highest private health insurance expenditures relative to Revenue collection is the process by which the health system total health expenditures (Sekhri and Savedof 2005). Asia, Africa, and Latin America, prepayment plans based Health systems have a limited set of mechanisms for col on the concept of pooling risk and resources have been lecting revenue, such as general taxation, mandated social developed for rural populations, groups in informal health insurance contributions (usually salary related and employment, or others without access to other health almost never risk related), voluntary private health insur insurance. Such schemes are based on community aflia ance contributions (usually risk related), or out-of-pocket tion, and the community is highly involved in managing payments from individuals. Most high-income countries rely on either general taxa Health or medical savings accounts: Not strictly a form of tion or mandated social health insurance contributions. Only a few countries, revenues was related to wealth (Gottret and Schieber 2006; including Singapore, China, and the United States, use Gupta et al. Decisions that afect the availability of health insurance in a country are important to all aspects of health service deliv Risk pooling ery but are largely outside the control of national medicine policies and essential medicines programs. As insurance Risk pooling spreads the fnancial risk associated with assumes a greater role in health care in many countries, health care among large groups. Pooling requires some however, understanding health fnancing, and specifcally transfer of resources (or cross-subsidization) from healthy insurance concepts, becomes increasingly important. Without such By their very nature, insurance schemes act as fnancing pooling, poor people are exposed to serious fnancial hard agents: they receive funds from employers, households, and ship when they get sick; the more extensive the risk pooling the government, and they use those funds to purchase health in a health fnancing system, the less individuals will have care for their benefciaries. Terefore, the main compo to bear the fnancial consequences of their own health risks, nents of insurance are collecting revenue, pooling resources and the more they are likely to have access to the care they and risks, and purchasing goods and services (Gottret and need (Carrin and James 2004). In a successful pooling scheme, contributions, whether Generally, insurance can be classifed as using either asingle through tax or insurance premiums, are not based on risk payer or multiple payer. If the pooling is voluntary, zation (usually the government) collects and pools revenues high-risk people and poor people will join the pool because and purchases health services for the whole population. All they see personal beneft, while healthier and richer people citizens are included in a single risk pool, and single-payer see less value for themselves (known as adverse selection). To provide services, most health systems many small organizations in revenue collection, pooling, use a variety of methods involving a mix of public, private and purchasing, restricts the efciency of all three tasks. Large pools are better ened capacity to bargain with providers regarding price, than small ones because they have a bigger share of contri quality, and opportunity of services. Purchasing agreements butions that can be allocated exclusively to health services. The purchasing process should include an ongoing while still ensuring that funds are sufcient to pay for ser search for the most cost-efective services to purchase, the vices without any risk sharing. In this case, each Three categories describe the financial relationship person constitutes a pool and thus has to pay for his or her between the insurer and the service providers in health own health services. The frst is reimbursement, where providers are paid afer they deliver the services. An example of the reim Prepayment bursement approach is called indemnity insurance, where no contractual arrangements exist between insurers and Prepayment is a feature of all types of health insurance. The Because it unlinks expected health expenditures from the second is contracting, where the insurers negotiate payment ability to pay, prepayment is a critical mechanism for attain agreements with certain doctors, hospitals, and health care ing health care equity. Without prepayment, consumers pay providers, to supply a range of services and possibly medi entirely out-of-pocket for health care, purchasing it, like any cines at reduced cost to those insured. The fairness of health the patient from the need to pay for health care up front fnancing is ofen measured by the amount of prepayment and also helps contain costs and control quality by giving required, because any out-of-pocket spending opens the the insurers direct purchasing power over providers. One example is a health als (risk subsidies) and high and low-income individuals maintenance organization, where the providers are salaried (equity subsidies). However, few systems are able to meet the entire cost of health care from the prepaid 12. Most require some type of co-payment systems for the use of health services or the purchase of medicines, which households must pay out of pocket. Market failure is the term used by economists to describe Prepayment without pooling simply allows for advance circumstances that constrain the smooth operation of the purchase of health services or purchase on an installment market (Normand and Weber 1994). Economists gener basis, such as in the example of health or medical savings ally agree that governments need to develop structures and accounts, which is presented later. In the case of health services, the major sources of market failure are Purchasing health care services the monopoly power of providers and ignorance and uncer tainty among consumers (Normand and Weber 1994). Purchasing is the process by which pooled funds are paid to providers to deliver health goods and services. All health Monopoly power of providers: To protect the public and fnancing systems face similar challenges in choosing which ensure a basic level of competence, entry into the health health services to buy or provide, who should provide them, care professions is restricted by licensing and other rules and which payment mechanisms are used. Tus, the health care government takes responsibility for collecting revenues for professions exercise monopoly power, limited to some the health system does not necessarily mean that it should degree if providers must compete against one another. Skimming reduces the equity benefts of insurance by In addition to the power exercised by professionals, a excluding those who are most in need. In the case of access to medicines, people demand caused by insurance coverage), and increases in rural areas may have little choice but to buy pharma of both the population in general and older populations. Tus the depend on health care professionals to inform them tendency is both for insureds to increase their use of of which services are appropriate and also to provide medicines and services and for providers to overprovide those services. The other models allow for greater cost con reinforces the monopoly power of professionals. When uncertainty and ignorance about the have to deal with misunderstanding of the insurance need or options for health care are combined with the concept by the public and by health providers. Members high cost of specifc types of care, market failure ofen may think that premiums are like deposits in a savings results. An insurance contribution is not a payment for such an environment, because no risk sharing occurs. Terefore, insurance systems they believe that they can claim only as much as they face several problems that can undermine the potential have contributed in premiums. Moral hazard: When members of a health insurance scheme use services or consume medicines more fre Country Study 12-1 shows some of the problems experi quently than if they were not insured, the phenomenon enced with a new insurance scheme in Bolivia that targeted is called moral hazard. Moral hazard ance organizations have developed measures to counter can be overcome by clearly describing the beneft pack moral hazard, adverse selection, skimming, cost escalation, age and trying to coordinate the co-payments and the and lack of familiarity with insurance. Policy makers, gov provider payment methods with incentives for necessary ernment insurance regulators, and insurers need to work care only. Adverse selection: this term describes the tendency for people at greatest health risk and people with chronic illnesses to join voluntary insurance programs, whereas 12. The Methods of provider payment are a crucial part of the design efect of adverse selection is to raise costs and reduce of all insurance schemes. Each method afects the admin the risk-sharing efect of insurance, which makes fnan istrative costs of the scheme according to the complexity cial sustainability much more difcult to achieve; for of approving claims and making payments. Most of those been addressed by vertical public health programs, deaths occur in the poorest sectors of society and are such as tuberculosis. Trough the program, women and children under fve Results suggest that the reforms further increased cover years of age receive free medical care and medicines for age of priority maternal and child services; for example, certain medical conditions that are common causes of between 1998 and 2002, pneumonia and diarrhea cov maternal and child mortality, such as unattended births erage for children under fve years increased from 69 for women, and diarrhea and acute respiratory illnesses percent to 100 percent and 29 percent to 43 percent, for children. Despite these increases, however, afer a few including primary care facilities and hospitals. However, this increase lowered the contributing 30 percent of national health spending. Designing payment mechanisms for medicines is more As an incentive to encourage rational medicine use and challenging because insurers fnd it more difcult to estab lower costs to the system, co-payments for medicines can be lish reimbursement schemes that cover all the places that set at diferent levels depending on whether the medicine is sell medicines, such as pharmacies, clinics, and informal on an essential medicines list or formulary, or whether it is drug sellers, than to administer payments to hospitals for generic or brand name (see Section 12. If co-payment charges are too high, however, they for a particular period of time. Administration is relatively can discourage people from seeking timely care, which can simple and inexpensive. Of course, not explicitly defned, meaning that providers have some cost sharing is also likely to be detrimental to poor peoples fexibility in terms of what they provide. Budgets can be set for providers, which, lessly transfer patients to higher levels of care. Budgets can also be among providers can decrease the tendency to under earmarked specifcally for medicines and supplies. Similar to produce, because poor service may cause patients to change what happens with use of capitation, savings occur because providers. Providers ability to contain overall costs, though, is limited if the budget is insufcient and results in others Fee for service is a payment mechanism in which the insurer having to provide the necessary care. However, this method of payment may also encourage an Salary overdelivery of health services (supplier-induced demand). Health workers employed by institutions are usually paid Wherever fees depend on medicine sales, the number of a salary. Salaries provide a neutral incentive to providers medicines prescribed increases. However, as with both bud may decrease the demand and counteract the overdelivery gets and capitation, salaried employees can sufer from low of services. When the institution is part of the gov Case payment ernment health system, low motivation can infuence pro viders to seek better remuneration in the private sector or An important example of a case payment is the diagnosis to moonlight in the private sector. To improve productiv related group payment method, wherein health facilities or ity, performance-related conditions and incentives can be providers are paid an inclusive fat sum for a patients treat implemented in addition to salaries. Case payments can be easier to administer than reimbursement from an itemized list, and requiring docu 12. According to the insurance concepts previously Payment for performance (incentive payments) discussed, pharmaceuticals might not be a top priority for insurance coverage: common illnesses for which medicines In a variation on fee for service or as an adjunct to capitation, are needed occur frequently, patients and providers may doctors or health facilities may be paid or partly paid based reinforce overprescription and overuse of medicines, and the on whether they achieve predetermined quality-based per potential for fraud and abuse is substantial. Such indicators may include appropri schemes will incorporate medicines as part of a comprehen ate prescribing of antibiotics, immunization coverage, blood sive care package, others will compensate for them sepa pressure screening, or cervical smears. However, there are strong arguments for including medi cines in insurance schemes. First, pharmaceuticals are an Budget transfer essential component of modern health care.
The peritoneum schema includes omentum and mesentery primary sites and all sarcomas in the range 8800 to 9852 except gastrointestinal and endometrial stromal sarcomas (8935 8936) and Kaposi sarcoma (9140) treatment for pink eye cheap combivent online visa. It should be noted that stage grouping uses essentially a two tier system 3 medications that cannot be crushed buy combivent american express, where grade 1 is categorized as low grade and grades 2 and 3 are categorized as high grade medicine wheel teachings order 100mcg combivent with amex. Do not code well differentiated or poorly differentiated or similar terminology in this field treatment 34690 diagnosis order combivent with a mastercard. If there is no biopsy/resection or there is no microscopic examination of tissue from the primary site medicine 5277 combivent 100mcg mastercard, use code 998 medications for ibs discount combivent 100 mcg on line. This is not the same as perineural invasion, which is a site-specific factor for skin cancers and other schemas. Use code 998 when there is no microscopic examination of tissue from the primary site. Pathologic confirmation of metastatic bone involvement is coded in site-specific factor 4. All other categories of gender (codes 001, 003, 004, 009 and 100) present the Peritoneum schema to the abstractor. In histology, the standard or routine stain is the hematoxylin and eosin stain, better known as the H&E stain. With rare exceptions, every specimen being examined will first receive an H&E stain to give the laboratorian a visible look at the nucleus of the cells and their present state of activity. With most disease states there is abnormal growth and/or division in the nucleus of the cells. The hematoxylin and eosin stain uses two separate dyes, one staining the nucleus and the other staining the cytoplasm and connective tissue. This counterstain acts as a sharp contrast to the purplish-blue nuclear stain of the nucleus, and helps identify other entities in the tissues such as cell membrane (border), red blood cells, and fluid. Immunohistochemistry is an additional test performed by the pathologist on lymph nodes that are pathologically negative on standard H&E stains. The group numbers are for convenience in using this chart only, and do not correlate with any anatomic groups of nodes. Fixed and matted ipsilateral axillary nodes None; does not apply 510 098 987 987 clinically, patient had pre-op chemotherapy. Axillary nodes clinically positive, patient None; does not apply 600 098 987 987 refused further workup. Cytokeratin H&E neg, molecular 000 000 002 002 stain showed clusters of tumor cells in the node studies done, pos for up to 0. Path report, final diagnosis: Lymph Nodes: H&E pos for 150 001-097 987 987 one of three sentinel lymph nodes positive for micromets (for this capsular micrometastases. The following information applies to both Estrogen Receptor Assay and Progesterone Receptor Assay. Example 1 Test Name Staining Percent Result Assay Type Intensity Positive Average (%) Estrogen Receptor 3+ 72 Positive Progesterone Receptor 3+ 57 Positive Example 2the neoplastic cells show mild (1+/4+) cytoplasmic staining with the estrogen receptor marker. Therefore, the code for borderline will rarely, if ever, be used for diagnoses 2010 forward. The new guidelines state that any test which results in 1% of the cells staining positive is a positive test. This field does not affect stage grouping, but can be used if needed by a researcher to analyze differences in outcomes within a T category. The score based on three factors: degree of tubule formation (histologic grade), mitotic activity, and nuclear pleomorphism (nuclear grade). Each of the factors receives a score of 1, 2, or 3, based on specific pathologic criteria. Clinical-only diagnoses should be coded as 998 reflecting the fact that there is no histologic specimen to score. In the absence of the local doctors interpretation, look on the actual lab report for that particular labs reference values and use that information to assign the appropriate interpretation code. The codes for interpretation are similar to other site-specific factors that are evaluated as positive/elevated, negative/normal, borderline, and so forth. Usually, the results will be either positive or negative, because if the result of counting the mean number of gene copies per cell from 30 cells is between 4. Amplification: >5 signals/nucleus, or cluster of amplified signals/nucleus in >50% of tumor cells. Younger women, African American women, and Hispanic women are more likely to be triple negative than older women and Caucasians, meaning that they are unlikely to respond to hormone therapy or Herceptin as part of their breast cancer treatment. Recent research efforts have concentrated on the identification of additional parameters allowing individual risk assessment and stratification of patients for targeted therapies, since traditional prognostic factors are not sufficient to predict metastatic relapse and treatment decisions are still mainly based on statistical risk parameters. In this three-digit field, the first digit codes whether the test was negative (0), positive (1), or borderline (2). If distant metastasis is coded as 000 (no positive metastasis), this field must also be coded to 000. Other names: genomic profiling, Oncotype Dx, MammaPrint, multigene testing, multigene assay, microarray assay, molecular diagnostics for treatment planning Multigene testing is usually done for node-negative patients to predict risk of recurrence within a specified time period or to predict the likelihood that the patient will respond to specific types of chemotherapy. A recurrence score is generated that predicts the risk of recurrence at 10 years for women treated with tamoxifen. This tissue must be collected in a kit and received by the company within 5 days from excision. Code any statement of Paget disease, clinical or pathologic, giving priority to the pathologic assessment. If physical or pathologic examination of the breast and nipple is negative or if Paget disease is not mentioned, code as 000. Many of the site-specific factors are the same for multiple primary sites, but the numbering of the site specific factors differs, as shown in Table I-2-11. In English, the organization is the International Federation of Gynecology and Obstetrics. Regional Lymph Nodes for can be coded from the pathology report, imaging or other Female Genital Organs information in the record. For the status fields, the basic Adapted from: Ovary and Primary Peritoneal codes are 000 Negative, 010 Positive, and 998 Lymph Carcinoma. Higher codes take priority over lower codes if multiple assessment methods were used. For all lymph node fields, code statements by the clinician or pathologist as appropriate. Endometrioid carcinoma is a hormonally dependent gland-forming carcinoma (adenocarcinoma). The most common non-endometrioid histology is papillary serous (10%), followed by clear cell (2% to 4%), mucinous (0. Some non-endometrioid endometrial carcinomas behave more aggressively than the endometrioid cancers, and even women with clinical stage I disease often have extrauterine metastasis at the time of surgical evaluation. Therefore, when technically and medically feasible, comprehensive surgical staging is helpful for women with non-endometrioid endometrial cancer histology. The term non-morular in the codes below means that the nodular structures are not present. Do not translate a verbal grade (well, moderately, or poorly differentiated) to code this field. The less left behind, the more likely the patient will respond well to adjuvant chemotherapy. This site-specific factor captures two pieces of information about residual tumor: residual tumor volume (amount) and whether the patient had chemotherapy. Information about residual tumor volume will be in the operative report; information about postoperative chemotherapy will be elsewhere in the medical record or physician notes. Two pieces of information are captured about residual tumor: the location of the residual tumor and whether the patient had chemotherapy. For example, code 030 is ovary (code 010) plus fallopian tube and/or uterus (code 020). Then the next code in the list includes all organs mentioned in the previous description and the patient received chemotherapy. Code 180 means that there was residual tumor on the diaphragm and one or more of the previously listed organs. In a code where multiple organs are described, such as 020 or 050, it is not necessary that all listed organs be involved with residual tumor. Code the location of the primary tumor within the fallopian tube if stated in the medical record. The eight risk factors and their point scores are shown in Table I-2-12, which lays out in table format the wording in the note for this site-specific factor. Carcinomas in the morphology code range 8000 8576, specialized gonadal neoplasms, and mixed complex and stromal neoplasms (except gastrointestinal stromal tumors) are coded with the same staging criteria for female patients as ovarian cancer in the PeritoneumFemaleGen schema. These sites will be discussed in order of their frequency of occurrence: prostate first, then testis, penis and scrotum. This site-specific factor is a 3 digit field with an implied decimal point between the second and third digits. In this three digit field, the tertiary pattern value is coded in the middle digit in the range 010 to 050. A diagnostic procedure can take as many as 20 or more core biopsies to determine the extent of the cancer within the prostate. Site-specific factor 12 captures the number of cores that contained cancer, and site specific factor 13 captures the number of cores that were examined. If the number of cores positive or cores examined is not documented in the record, code 991. Note: Make no assumptions about the number of cores positive or examined based on the number of areas biopsied within the prostate (laterality, lobes, apex, base, or mid-prostate), because several cores may be taken from each area. Site-specific factor 14 documents the location of tumor discovered on core biopsy. A radical orchiectomy is defined as complete removal of the testicle, epididymis, and spermatic cord to the level of the internal inguinal ring, either as a diagnostic procedure or as treatment. Unless the operative report says that the cord was not removed, assume that the procedure was a radical orchiectomy. Read the descriptions carefully, as the ranges change substantially in the upper categories. This requires serial tumor markers that Version date: 25 January 2010 I-2-100 Version 02. The only exception is if the serum tumor markers were normal prior to orchiectomy; if so, code as 000. The pathologist should report the percentage of poorly differentiated tumor, even if the majority of the tumor is well or moderately differentiated. Use code 000 if there is a statement that poorly differentiated tumor is not present or that no poorly differentiated tumor is identified. If the pathology report mentions poorly differentiated tumor but does not give a percentage, use code 990. This site-specific factor applies to carcinomas only; rare sarcomas of the kidney should not be coded in this field. The Fuhrman grade originally published in 1992 is unique to renal cell carcinomas. Code the Fuhrman nuclear grade as stated in the pathology report in the range 010 (Fuhrman grade 1) to 040 (Fuhrman grade 4). Do not use the Fuhrman nuclear grade to code the fields Grade Path System and Grade Path Value. For flat urothelial lesions, this terminology distinguishes dysplasia (low grade intraurothelial neoplasia), which is not reportable to population-based cancer registries, from urothelial carcinoma in situ (formerly called transitional cell carcinoma in situ), which is reportable. This site-specific factor applies to urothelial (transitional cell) carcinomas only. This two-grade system can also be used to code the fields Grade Path System and Grade Path Value. This site-specific factor adds prognostic information by coding the size of the metastasis within the lymph node. Tumor grade is the most important prognostic indicator for response to therapy and outcomes for brain and spinal cord tumors.
Even if reading is most current edition available when using secondary or ter limited to journals in a specifc area of interest and expertise medications you can take while nursing cheap combivent 100 mcg on line, tiary sources symptoms 8 days after iui discount combivent online mastercard. All informa journals peer-review processes and assume that the process tion sources have limitations medications with weight loss side effects combivent 100mcg on-line, and medicine information will identify any problems with the studies medicine woman discount combivent online amex. Information should include on medical products and the Internet to help people active ingredients symptoms gallstones combivent 100mcg without a prescription, other ingredients treatment narcissistic personality disorder 100mcg combivent with visa, side efects, obtain reliable, independent, and comparable informa interactions, how to use a product, how to store it, tion. The Internet is a valuable source of information, but cal products on the Internet. If the by the service, and are these resources the ones that publication is reporting proceedings from a confer are essential for the particular purpose However, considering the source of a study or paper conclusions survive the peer-review process, some reader is useful when determining quality. A number of respected will undoubtedly write to the journal editor and state his medical and pharmacy journals whose high standards for or her observations or concerns. Reputable journals read acceptance and publication make it unlikely that a research ily share this type of correspondence, ofen allowing the article containing erroneous, fraudulent, or misrepresented authors of criticized articles the opportunity to respond. Annex Information published in journals without a strong review 34-1 lists some English-language journals that have strong process needs to be more carefully scrutinized. One way to monitor such problems is to read the determine what biases, if any, exist. Finding information on clinical trials has been process is, if any, for acceptance of an article is a good idea. For example, the Clinical trial databases and unpublished reports (known Duke University Medical Library has a guide to searching as gray literature) are also considered primary literature. The panies or researchers may elect not to publish results because reader needs to carefully interpret unpublished data, how the fndings were negative. This decision presents an ethical ever, because there may be negative underlying reasons why problem in that negative fndings, although potentially det the data have not been published in peer-review journals. Secondary and ter valuable in making evidence-based decisions on the use of tiary information resources are essentially derivations of Box 34-3the Cochrane Collaboration The Cochrane Collaboration was established in 1993 in Although the Cochrane Collaboration has centers in England to support the systematic, up-to-date review and Australia, Canada, Denmark, England, Italy, and the synthesis of scientifc research, which can be used to help United States, it is beginning to infuence far-reaching people make well-informed decisions about health care. Afer extensive peer review, the documents are are available to the public, but most full-text documents published and updated periodically. Access to the reviews is steadily improving for people in low and middle-income Cochrane reviews cover treatments for many diferent countries through free national subscriptions or global diseases. Co-trimoxazole is cheap and efective against a wide range of organ E-mail: secretariat@cochrane. Some review articles summarize the that they can select topics of national relevance and use the results and conclusions of a number of reports from the pri national language. Tertiary references, written by individuals or groups, are Systematic reviews of data from multiple trials addressing ofen developed with the input of consultant authors and the same research question (meta-analyses) are particularly reviewers and may be widely peer reviewed. The Cochrane Collaboration (see Box 34-3) under the more thorough the review process is, the more sound takes this type of work. In many countries, the most Bibliographic, abstracting, or indexing services pro widely available tertiary resources are formulary manuals vide listings or compilations of published articles. Some and standard treatment manuals produced by the health list the addresses of the principal authors; others con system. Tese important resources are discussed in Chapter tain abstracts of articles, along with key words or subject 17. Examples of such services include information sources are those that report the consensus of PubMed, Embase, International Pharmaceutical Abstracts, experts, a process that involves a high level of scrutiny and Index Medicus, Excerpta Medica, and the Iowa Drug feedback. In most instances, consensus journals and may, for instance, omit letters to the editor. The consensus documents Not relying entirely on one secondary source is therefore developed by the U. Drug bul letins can have a variety of sponsors, such as government Manufacturer-provided medicine information agencies, professional bodies, university departments, philanthropic foundations, and consumer organizations. Information provided by pharmaceutical companies is so They are published in many countries, sometimes free commonly available and widely used that it warrants a sepa of charge, and many are highly respected because of their rate discussion. Examples in English are Drug and product labeling approved by a countrys regulatory agency, Terapeutics Bulletin (United Kingdom), Medical Letter textbooks, and journal reprints, or promotional informa on Drugs and Terapeutics (United States), and Australian tion. Prescrire International is available in panys product is better than anothers or how a new product both French and English. National drug bulletins appear in can treat a serious or not-so-serious medical condition. Many pharmacy and medical educators have recognized In 2005, an international cross-sectional survey, Edu the need for education about medicine promotion, but cational Initiatives for Medical and Pharmacy Students they frequently mention a lack of integration into the about Medicine Promotion, examined the extent to main curriculum and inadequate time allocation as bar which students are educated about the pharmaceutical riers to a successful program. The results are based In some cases, students themselves are taking an active on a survey of 228 pharmacy and medical school educa role in opposing the infuence of medicine promotion. In 2002, the American Medical Student Association In the survey, nearly three-quarters of educators reported launched its PharmFree campaign, which educates that education about medicine promotion is part of their medical students about the infuences of the pharma required curriculum but that most students devoted less ceutical industry on medical training and the problems than one-half day to this topic. The regional breakdown with using biased industry-based information to choose is illustrated in the table below. Educators report of the role of medicine promotion in international pharmacy and medical school curriculum Eastern Western Mediterranean southeast role of medicine promotion Europe (%) americas (%) Pacifc (%) africa (%) (%) asia (%) Promotion is part of curriculum 81 83 67 86 70 91 Promotion is part of required 75 64 81 85 65 56 curriculum One-half day or less spent 30 32 39 25 20 20 on promotion Ten or more hours spent 40 34 32 50 45 44 on promotion terms of source and presentation. The information developed as by pharmaceutical manufacturers varies considerably from part of a new medicine approval process by a countrys drug country to country, depending on a governments regula regulatory authority has been thoroughly reviewed and tory requirements and its ability to efectively monitor and should accurately refect a products basis for approval. Approved product information guides prescribers, characteristics and use that are based on approved product dispensers, and patients on use of a particular medicine and information. In the United States, the most common refer defnes what the manufacturer can legally say (advertise) ence of this type is the Physicians Desk Reference, where about its product. This information includes, for example, pharmaceutical companies pay to include their products the medicines approved indications for use, precautions, (making it marketing focused). In the United Kingdom, potential adverse efects, and dosing, as well as the products the Association of the British Pharmaceutical Industry strength, composition, and packaging and storage require Data Sheet Compendium is commonly used; and in many ments. Because this information brief sets of information for products marketed in that is approved by the drug regulatory authority, it carries sig country or region. The limitations of these types of publi nifcant impact both clinically and legally. A new statement on ethical standards in packaging and labeling, patient information, criteria for medicine promotion was adopted in 1988. It includes download the approved scientifc data sheet and should be fully able resources related to promotion to consumers, legible. Advertisements to the public should be for non promotion in health education, and drug industry prescription medicines only. Lobby for Appropriate Marketing, is a nonproft They should make unbiased information on products advocacy group that initially focused on revealing available. The criteria also state that promotion information includes more than 19,000 providing free samples of nonprescription medicines items. Do they come from peer-reviewed jour comes related to the use of a medicine (such as side nals They are not comprehensive information treatment guidelines, and be involved in the production of sources. Although most countries have regula support information access and dissemination. A multinational advocacy is usually an emergency service requiring rapid response. However, health care professionals or patients or passive duties include providing information for people using manufacturer-supplied sources need to recognize the who call or come to the center with questions. Although potential for bias and make a judgment about the materials this function is important, it certainly should not defne the value (see Box 34-7). A centers efect will be greater if it functions proactively by reaching out with medicine information for people who need it, in a format that is con 34. This task will be easier if a medicine information center information service is based on a cooperative model, involv ing all health care disciplines and using existing resources A medicine and therapeutics information center is a vital to the greatest extent possible. In a be driven by the needs and expectations of its users but also small country with limited means, this center may be a should work to create demand and raise expectations. Location within a hospital, university, or national essential medicines program, provide support for other academic institution provides a network of medical development and maintenance of formulary and standard disciplines that can support and enrich the work, allowing 34. When medicine drug regulatory authority, pharmaceutical approval unit, or information specialists are not available, a medical doc quality-control laboratory). The user population for any information service is efcient of these resources are the computer databases, cost primarily clinicians, and expertise in pharmacotherapy is may limit their availability. Some appropriately trained person is not available, every efort print and electronic databases can be accessed free of charge. Many and microfche) scientifc journals are now available via open access or are free of charge. A list of many such journals can be found at the Directory of Open Access Journals. Because acquiring and updating information is costly, establishing a link to a medical library is very important. If Internet training health professionals and regularly evaluating the access is unavailable or unreliable, however, or if the costs of performance of the centers staf. Although most coun The center implemented a nationwide system for spon tries separate these activities, this integrated model was taneous reporting of adverse drug reactions in 2007, driven by the potential synergies between the two ser through which it collects reports from health care pro vices, opportunities for leveraging resources, and human viders and the public of adverse efects of medicines. Tese periodicals should promote rational medicine publication development, and sustainability planning and therapy and appear at regular intervals, ranging from weekly funding. A large medicine informa assessments of medicines and practical recommendations, tion service should ideally have a career structure similar to based on a comparison of treatment alternatives and on the those of academic or educational institutions. Understanding the reasons for prescribing behavior: As men tioned in Chapter 29, providing information alone does Evaluation not change undesirable behavior. Understanding the rea sons for the behavior is a necessary frst step in develop Ongoing monitoring and evaluation is particularly impor ing appropriate messages. Monitoring should be built in from the start and Emphasizing and repeating only a few key messages: If too should include documenting the questions asked, responses many ideas are brought up in the bulletin, none will be provided, references used, complaints and compliments absorbed. A few messages that are the focus of the bulle received, timing of responses, and services provided (such tin and are repeated are more likely to be retained. The queries should be ana Capturing attention with headlines and visually appealing lyzed, and the results summarized in the annual report. In illustrations: An efective bulletin grabs the readers atten addition, periodic input from users of the medicine infor tion with attractive graphics that emphasize key mes mation service should be sought through personal contacts, sages. Keeping text brief and simple: Although readers of the bul this information can help the centers manager make letin may be well educated and knowledgeable, a bulletin good decisions about future programs and budgeting. For example, the International Pharmaceutical an article in the medicine information newsletter or bulle Federations Pharmabridge program helps provide tin. Health Programme also allows developing countries to use a large collection of scientifc and medical literature Sources of help who. Blue trunk libraries are available in English, cines projects, professional associations, university or other French, Portuguese, and Arabic. Collaboration between training programs, nongovernmental organizations, or a centers in developing and developed countries is very valu combination of several of these funding sources. Box 34-8 starting or strengthening a drug bulletin Drug bulletins are a fundamental tool for promoting pendent medicine information. Working closely with the rational use of medicines, and locally produced bulletins National Drug Committee, Kyrgyzstans drug informa are an efective approach to providing reliable and unbi tion center has taken on the important role of providing ased comparative information on medicines and thera independent medicine information to health care pro pies for prescribers, patients, and the public within the viders.
Buy combivent 100mcg cheap. SHINee - In My Room [Han & Eng].