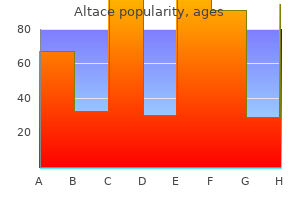
Altace
Mark J. Spoonamore, MD
- Assistant Professor, Clinical Orthopedic
- Surgery
- Medical Director, Center for Spinal Surgery,
- University of Southern California? Keck School of
- Medicine, CA, USA
In evaluating numbers of spontaneous reports against patient exposure 4 purchase altace 1.25mg with mastercard, different options are possible for the appropriate units; each has advantages and disadvantages heart attack karaoke demi lovato order altace 2.5mg without prescription. However arrhythmia used in a sentence buy altace visa, there is commonality across many countries for expedited reporting of serious unexpected cases blood pressure chart readings for ages purchase altace 1.25mg fast delivery, whether of local or foreign origin heart attack jack look in my eyes 1.25mg altace amex. Although considerable progress has been made toward international harmonization of requirements and practices hypertension medication discount 5 mg altace with visa, considerable work remains to eliminate inefficiencies and unnecessary differences so as to optimize the contributions of pharmacovigilance. It was attended by health professionals, researchers, academics, media writers, representatives of the pharmaceutical industry, drug regulators, patients, lawyers, consumers and international health organizations. High scientific, ethical and professional standards and a moral code should govern this activity. The inherent uncertainty of the risks and benefits of drugs needs to be acknowledged and explained. Decisions and actions that are based on this uncertainty should be informed by scientific and clinical considerations and should take into account social realities and circumstances. Flaws in drug safety communication at all levels of society can lead to mistrust, misinformation and misguided actions resulting in harm and the creation of a climate where drug safety data may be hidden, withheld, or ignored. Fact should be distinguished from speculation and hypothesis, and actions taken should reflect the needs of those affected and the care they require. These actions call for systems and legislation, nationally and internationally, that ensure full and open exchange of information, and effective standards of evaluation. These standards will ensure that risks and benefits can be assessed, explained and acted upon openly and in a spirit that promotes general confidence and trust. The following statements set forth the basic requirements for this to happen, and were agreed upon by all participants, from 30 countries at Erice: 1. Such information should be ethically and effectively communicated in terms 219 of both content and method. Facts, hypotheses and conclusions should be distinguished, uncertainty acknowledged, and information provided in ways that meet both general and individual needs. Education in the appropriate use of drugs, including interpretation of safety information, is essential for the public at large, as well as for patients and health-care providers. Drug information directed to the public in whatever form should be balanced with respect to risks and benefits. All the evidence needed to assess and understand risks and benefits must be openly available. Constraints on communication parties, which hinder their ability to meet this goal, must be recognised and overcome. Every country needs a system with independent expertise to ensure that safety information on all available drugs is adequately collected, impartially evaluated, and made accessible to all. Exchange of data and evaluations among countries must be encouraged and supported. A strong basis for drug safety monitoring has been laid over a long period, although sometimes in response to disasters. Innovation in this field now needs to ensure that emergent problems are promptly recognised and efficiently dealt with, and that information and solutions are effectively communicated. These ideals are achievable and the participants at the conference commit themselves accordingly. Details of what might be done to give effect to this declaration have been considered at the conference and form the substance of the conference report. Throughout the various meetings, concepts were presented and debated, drafts of proposals were reviewed and discussed, and two surveys of the industry were carried out (one on practices and experience in preparing periodic safety update reports (see Chapter 4) and the other on knowledge and use of patient exposure information (see Chapter 5)). The meetings subsequent to April 1997 were as follows: July 1997 (Geneva), November 1997 (New York), April 1998 (Paris), November 1998 (Philadelphia), March 1999 (Amsterdam), July 1999 (Berlin), and August 2000 (Barcelona). In May 1999 and February 2000, the appointed editorial committee for the report (A. Lumpkin) held meetings to resolve outstanding issues and design the overall report. However, it is common practice to rely on at least two such sources for literature searches. Perhaps the two most widely used general biomedical databases for this purpose are Medline and Embase. In addition there are several more general biological and scientific databases such as SciSearch, Biosis, and the Derwent Drug File. There are also specialized databases which deal with specific disease areas (such as CancerLit and AidsLine), or with the toxicological effects of drugs (ToxLine). Medline Medline is a vast source of medical information, covering the whole field of medicine including dentistry, veterinary medicine and medical psychology. The database covers clinical medicine, anatomy, pharmacol ogy, toxicology, genetics, microbiology, pathology, environmental health, occupational medicine, psychology, and biomedical technology, etc. The database corresponds to the printed publications: Index Medicus, Index to Dental Literature, International Nursing Index and various biblio graphies. It is also available in many manifestations on the World Wide Web, several of which are free to use. It features unique international journal coverage and includes many important journals from Europe and Asia not found in other biomedical database; overall coverage is approximately 4,000 journals published in 70 countries. The emphasis of the database is on the pharmacological effects of drugs and chemicals. Additional areas of coverage are human medicine and biological sciences relevant to human medicine, health affairs (occupational and environmental health, health economics, policy and management), drug and alcohol dependence, psychiatry, forensic science, pollution control, biotechnology, medical devices and alternative medicine. It indexes all significant items (articles, review papers, meeting abstracts, letters, editorials, book reviews, correction notices, etc. Some 3,800 of these journals are further indexed by the references cited within each article, allowing for citation searching. It selectively covers the worldwide pharmaceutical literature; papers chosen may cover the chemistry, analysis, pharmaceutics, pharmacology, metabolism, biochemistry, interactions, therapeutic effects and toxicity of a drug. Papers from over 1,150 scientific and medical journals and conference proceedings are included. Each year approxi mately 9,000 articles on adverse drug reactions are published in the scientific literature. All articles are sent to recognised authorities who critically assess the information and distil the key elements for inclusion. Speculative or unsubstantiated statements on the side effects of ethical drugs are not included. The database consists of bibliographic records referencing cancer research publications dating from 1963 to the present. In addition, proceedings of meetings, government reports, symposia reports, selected monographs, and theses are also abstracted for inclusion in the database. Even with a single company statement, however, there can well be debate and sometimes discrepant views between personnel within an organization as to what a safety data mean. In addition, 22 physician monitors enployed by either Glaxo or SmithKline Beecham Pharmaceuticals completed the exercise. Despite these efforts, reporting discrepancies within and between organisations are ocurring. These are felt to be not only due to cultural differences between organizations and regulatory agencies. In the absence of standardized guidelines, such opinions caused by a nonstandar dized view can lead to the same case history being reported to some regulatory authorities but not to others, even though reporting is based on the same reference data and similar regulations. No attempt was made to compare responses from regulators attending the meeting with the others, although it would have been interesting. As can be seen, there appears to be a different philosophy between Europe and the United States in the way the events are interpreted, particularly where the outcome is death (examples 6 and 9). Individuals in the United States would tend to report a fatality as an unlabeled event, whereas in Europe this is generally viewed as an outcome rather than a factor relevant to labeledness. For example, in Europe, 97% of the respondents accept that if myocardial infarction is to be labeled, death 230 due to myocardial infarction is also labeled. Only 43% of the respondents in the United States, however, would accept fatal myocardial infarction to be labeled if only myocardial infarction appeared in the labeling. Also of interest is that in Europe, rather than the United States, cyanosis secondary to hypoventilation was equated with respiratory depression (Example 8). As can be seen, medical opinion was unanimous among 22 medical monitors in only one example (Example 1) where the greater anatomical specificity did not affect the labeledness of lung fibrosis. Table 2 was designed to gather responses on whether certain medical events should be considered to be serious. For instance, in Example 1, total blindness for 30 minutes was considered to be serious in Europe, whereas in Example 3, mild anaphylaxis was thought to be serious in the United States. This is in contrast to the overall total of 95% who consider this event to be serious. The information found in Table 3 was designed to determine whether the respondents felt, based on the available case details, the case should be reported to regulatory authorities. The results suggest a fairly uniform transatlantic view about whether or not a case should be reported. Fewer in Europe than in the United States, however, would report a case where the reporter could not remember the age or even the sex of a patient (Example 167). Discussion None of the 30 examples surveyed achieved a totally unanimous view and so the guidelines presented below are all based on a majority verdict. To some extent the non-uniform opinion is surprising because of the relatively small number of individuals who took part in the survey, with many having a substantial amount of expertise in the area of drug safety. It appears that in many situations reporting is practiced according to medical common sense. It is believed that the newly proposed United States regulations, in the wake of fialuridine experience, should serve to move general opintion further toward reporting based on medical opinion. Worthy of comment is that the extra reporting is not always within the United States. For example, blindness for 30 minutes, respiratory collapse, and respiratory depression would be more frequently viewed as medically serious and would be reported more often in Europe. The United States reporting practice is more to view fatalities as unlabeled unless death is specifically mentioned in the label. Before suggesting a pragmatic way forward to best benefit from the harmonization initiatives, the following 20 guidelines are proposed. An unlabeled diagnosis which relates to a group of symptoms or signs which are labeled, the new case is not in itself labeled. For example, if anaphylaxis is labeled, then a report of a patient who experienced hypotension, wheezing, and urticaria would be considered to be labeled (69%). If a report is serious in the medical sense, even though it is not serious in the regulatory sense. On the other hand, a threat of suicide is not considered to be serious (83%), nor in itself is an emergency room or outpatient department visit (97%). If the investigator persists in specifying a case is drug-related, even though this view is medically nonsensical, the case should be considered drug-related and reported to the regulatory authorities. Spontaneously reported cases should always be considered to be possibly drug-related, even if an alternative explanation is given by the reporter (72%). The overall majority of individuals surveyed would not report to regulatory authorities a case where the details of a specific patient could not be recalled. In the United States, however, 72% would report the unidentified patient and 59% the series of eight cases. In those instances where the brand name of a generic drug is stated to be unknown, the case should be processed and reported to regulatory authorities by the company which becomes aware of the adverse event (70%). In the United States, this obligation generally falls to the original brand name manufacturer of the drug. Even with a single company statement, however, there is often debate and sometimes discrepant views between personnel within the organization.
Papulonodular lichenoid hand pseudolymphomatous reaction at the injection site of hepatitis B virus vaccination hypertension jnc 8 cheap 2.5 mg altace free shipping. Churg-Strauss vasculitis with brain involvement following hepatitis B vac cination arrhythmia exam purchase altace with a visa. S Diagnostic methods Skin tests Intradermal tests with undiluted human serum albumin lead to false positive results blood pressure while pregnant purchase altace in united states online. Tryptase: elevated at the time of reaction S Mechanisms IgE-mediated hypersensitivity is suggested by positive immediate skin tests and evidence of specific serum IgE blood pressure readings cheap altace 10 mg without a prescription. Repeated administration of recombinant human serum albumin caused no serious allergic reactions in patients with liver cirrhosis: a multicenter clinical study arrhythmia during exercise 1.25mg altace visa. Anaphylactoid shock in a patient following 5% human serum albumin infusion during off-pump coronary artery bypass grafting hypertension medications buy altace 5 mg low price. Uneventful plasma exchange with albumin replacement in a patient with a previous anaphylactoid reaction to albumin. S Diagnostic methods Skin tests Prick tests with pure vaccine and intradermal tests 1/100 are usualy negative. Specific IgE (IgE immunoblot): a protein band at 100 kDa (gelatin), a protein band at 68 kDa (hemag glutinin from influenza vaccine), a protein band at 45 kDa (ovalbumin) in a patient with anaphylaxis. S Mechanisms Egg allergy (ovalbumin): the content of ovalbumin/ovomucoid is variable: 0. S Management (controversial) In patient with egg allergy and skin tests positive to vaccine: vaccination in a 2 dose protocol at 30 min interval if the vaccine preparation contains no more than 1. Mild: headache, flushing, low backache, muscle pain, nausea, chills, abdominal pain. IgG anti-IgA antibodies are detected in 22% of patients with common variable immunodeficiency, and in 20 to 60% of patients with selective IgA deficiency. Anti-IgA antibodies are found more frequently in patients with combined IgA and IgG2 subclass defi ciencies. IgA antibodies are class-specific, subclass-specific, antiallotypic, antiisoallotypic, or of limited speci ficity. S Mechanisms Formation of immune complexes between antibodies in intravenous immunoglobulins and micro bial antigens in the recipient with subsequent complement activation. Use of IgA-depleted intravenous IgG preparations until the activity of anti-IgA decreases significantly or becomes undetectable. Ex-vivo pretreatment of intravenous immunuglobulin preparation containing less than 0. Immune tolerance induction in patients with IgA anaphylactoid reactions following long-term intravenous IgG treatment. A Baboon syndrome induced by intravenous human immunoglobulins: report of a case and immunological analysis. Unusual skin reactions after intravenous infusion of polyvalent immunoglobulin: 3 cases (Article in French). Vasculitis, Henoch-Schonlein purpura, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, Gianotti-Crosti syndrome, Mucha-Haberman disease. S Diagnostic methods Skin tests Prick or intradermal tests in egg-allergic individuals have been debated; several studies have found poor positive and negative values. Extra precautions including continuous observation for 20 min after vaccination, with further moni toring of cardiorespiratory parameters to a total of 2 hours are needed if there is a history of any cardiorespiratory symptoms or signs after egg ingestion or active chronic asthma is present. Recommendations for administering the triple viral vaccine and antiinfluenza vaccine in patients with egg allergy. Erythema multiforme following live attenuated trivalent measles mumps rubella vaccine. Prevalence of anti-gelatin IgE antibodies in people with anaphylaxis after measles-mumps-rubella vaccine in the United States. Pytiriasis rosea-like eruption due to pneumococcal vaccine in a child with nephrotic syndrome. Anaphylaxis to the 23-valent pneumococcal vaccine in child: a case-control study based on immediate responses in skin tests and specific IgE determination. Specific serum IgE (immunofluorescence): the specificity of this method has been confirmed by solid phase binding of the vaccine to antigens (19 out of 21 cases of urticaria). The vaccine should be prepared without b-propiolactone (inactivation with formalin or tributyl phosphate only). Boosters should only be administered to risk-group patients the use of the intradermal route for both primary and booster injections may result in lower rates of reactions. Safety and immunogenicity of Lyssavac Berna human diploid cell rabies vaccine in healthy adults. Risk factors for systemic hypersensitivity reactions after booster vac cinations with human diploid cell rabies vaccine: a nationwide prospective study. IgE and IgG antibodies to beta propiolactone and human serum albu min associated with urticarial reactions to rabies vaccine. Immunologic studies in subjects with a serum sickness-like illness after immunization with human diploid cell rabies vaccine. Systemic allergic reactions following immunization with human diploid cell rabies vaccine. S Incidence Local reactions after booster injections: pain and tenderness: 50 -85% erythema and edema: 20 30% marked swelling: 2% abscess: 6 to 10/million doses. Positive in allergic patients but also positive in 8 to 63% of non-allergic vaccinated patients. Use an isolated tetanus toxoid which is less reactogen than associations (diphtheria/ tetanus) Desensitization has been reported to be effective. Extensive swelling reaction associated with diphtheria and tetanus toxoids and acellu lar vaccine. Immediate allergy to tetanus toxoid vaccine: determination of immunoglo bulin E and immunoglobulin G antibodies to allergenic proteins. Anaphylactic reaction to diphtheria-tetanus vaccine in a child: specific IgE/IgG determinations and cross-reactivity studies. Allergy to multivalent vaccines in children: a study of 30 cases using immediate, semi-late and late skin test responses, specific antibody assays, and challenge with monovalent and bivalent vaccines (Article in French). Immunization against tetanus in a hypersensitive individual using a graded dosing regimen (letter). Yellow fever desensitization to an antiamaril 17 D vaccine performed on a patient with anaphylaxis to eggs (Article in French). Cross-reactivity may exist between calcipotriol and other vitamin D3 analogue: tacalcitol and calcitriol. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. S Diagnostic methods Skin tests Prick tests: positive with pure hydroxocobalamin in a few cases Intradermal tests: positive at 1/100 to 1/10 dilution. S Mechanisms the vitamin itself, the preservatives (benzyl alcohol) or some contaminants may be involved. Contact dermatitis due to the cobalt ring contained in this vitamin has been reported. Possible IgE-mediated hypersensitivity (positive skin tests, specific histamine release). S Management Cross-reactivity between hydroxocobalamin and cyanocobalamin has been described but is not always found. In patients with hydroxocobalamin allergy, if skin tests are negative cyanocobalamin can be used in increasing intramuscular doses (0. Desensitization in patients allergic to both hydroxocobalamin and cyanocobalamin may be perfor med. Allergy to vitamin B12: two cases of successful desensitization with cya nocobalamin. Adverse reactions to vitamin B12 injections due to benzyl alcohol sensiti vity: successful treatment with intranasal cyanocobalamin. Folinic acid (5-formyltetrahydrofolate) bypasses the reduction steps required for folic acid. S Diagnostic methods Skin tests: positive in a patient to folic acid and other folate analogues. A diet rich in natural folates (pteroylpolyglutamates) appears useful as a management strategy to provide adequate nutrition to patients with folic acid hypersensitivity. In vitro demonstration of IgE antibody to folate-albumin in anaphylaxis from folic acid. S Incidence One case described S Clinical manifestations General: anaphylactic shock. Intradermal skin tests: positive S Mechanisms Possible IgE-mediated hypersensitivity. S Diagnostic methods Skin tests A few cases of positive skin prick tests or intradermal tests (0. S Management Administration of parenteral thiamine only when required (thiamine deficiency). Specific IgE and IgG serum antibodies to thiamine associated with anaphy lactic reaction. Pyridoxine is widely used in the preparation of medica tions and cosmetics (hair lotion). Occupational and systemic contact dermatitis with photosensitivity due to vitamin B6. Persistant reactions are possible despite treatment with topical steroids (several months). Reaction resembling localized scleroderma or morphea (more rare): onset from 2 months to 1. Contact dermatitis: occupational contact with vitamin K3 (in pig feed, pharmaceutical factories and laboratories, and in veterinary laboratories) Urticaria, diffuse maculo-papular rash: a few cases have been reported. Patch tests may be positive in patients with an eczematous localized site reaction (sometimes delayed after the 4th day). Intradermal tests may be positive in eczematous localized site reactions, with an eczematous reac tion developing in 48 to 72 hours. Patch tests may be negative and intradermal reactions positive in the same patient. The phytyl moiety contained in phytomenadione, but not in other forms of vitamin K, might be the epitope. Cross-reactivity between vitamin K3 and K4 has been described, but not between vitamin K1 and other vitamin K derivatives. Intravenous vitamin K should be limited to patients with serious hemorrhage that is secondary to a relative or absolute deficiency of vitamin K. The incidence of anaphylaxis following intravenous phytona dione (vitamin K1): a 5 year retrospective review. Cutaneous hypersensitivity reactions to vitamin K: 2 case reports and a review of the literature. S Diagnostic methods Skin tests Prick tests: 10 mg/ml (ranitidine, nizatidine) Or 0. S Mechanisms IgE-mediated hypersensitivity (positive skin tests, specific serum IgE, leukocyte histamine release). Cross-reactivity among histamine H2 receptors antagonists exists (ranitidine/nizatidine). Use another H2 antagonist if necessary (after negative cutaneous testing and oral challenge). Drug eruption caused by ranitidine hypochloride (Zantac*) which showed a strong reac tion in a drug-induced lymphocyte stimulation test. Anaphylactic reaction to drugs commonly used for gastrointestinal system diseases 3 cases reports and review of the literature. Proton pump inhibitors are widely used in the treatment of peptic ulcer and gastroesopha geal diseases.
For each of the test conditions arrhythmia in fetus cheap altace 5mg on line, samples were removed blood pressure 7545 purchase altace 5mg online, include pH buffers blood pressure medication that starts with m order 2.5 mg altace with mastercard, energy substrates arrhythmia qt interval prolongation purchase altace 10 mg mastercard, free radical scavengers blood pressure up and down purchase genuine altace on line, and osmotic/ thawed hypertension pulmonary buy altace 1.25mg on-line, diluted with media and plated. Cells were assayed for metabolic activity following 24 hours recovery post-preservation. Each of the major proteinacious technical team at (800) 325-0250 or by e-mail at clintech@sial. Each of the major proteinacious chamber contains Histopaque-1077 which allows the addition of anticoagulated whole blood without risk of mixing with the separation medium. On centrifugation, erythrocytes & components was screened by substitution in samples of the complete granulocytes descend through the frit to pellet below the Histopaque-1077. If the thawed medium will not be used allows the addition of anticoagulated whole blood without risk of mixing within a few days it is recommended that the medium be refrozen in with the separation medium. On centrifugation, the whole blood descends working aliquots to avoid repeated free-thaw cycles. The nature of the supplement may affect a clear separation of the blood components. Used to create a density medium for the purification of lymphocytes and Each tube contains 3 mL Histopaque 1077-1 and will separate 3-6 mL of other mononuclear cells. This medium facilitates rapid recovery of viable lymphocytes from small volumes of whole blood. It may also be employed as the initial Ficoll isolation step prior to enumeration of T-, B-, and null lymphocytes. Most extraneous platelets are removed by low speed Dialyzed and lyophilized centrifugation during the washing steps. After centrifugation, use a Pasteur pipette to aspirate the upper layer to ship: ambient store at: room temp within 0. With a Pasteur pipette, carefully transfer the opaque interface to a clean, F8016-500G 500 g conical centrifuge tube. Ficoll is a hydrophilic neutral highly branched polysaccharide use to establish 8. Assays for solution is added directly to the cells, no pre-mixing of components is cell proliferation may monitor the number of cells over time, the number of required. This technique has Cell Counting Kit been utilized as an alternative to 51Cr release for cell mediated cytotoxicity assays, as well as conventional cytotoxicity resulting from interaction of a test sufficient for 500 tests material with the cell. Therefore, it allows the users to obtain highly reproducible and accurate cell For spectrophotometric measurement of cell viability by mitochodrial proliferation assay results. Replicative senescence is a growth-arrest state associated with loss of division For spectrophotometric measurement of metabolic activity of living cells. For spectrophotometric measurement of cell viability by mitochodrial dehydrogenase. Useful as an indicator for preparing neutral red paper, and as a biological visual transition interval. The bioreduction of [8005 03 6] the dye reduces the amount of the oxidized form (blue) and concomitantly powder, BioReagent, suitable for cell culture increases the fluorescent intermediate (red). It is a vital stain that is not absorbed by healthy viable ring by dehydrogenase enzymes. When cells are damaged or dead, trypan blue can enter the cell solubilized using isopropanol or other solvents and the dissolved material is allowing dead cells to be counted. The method is sometimes referred to as measured spectrophotometrically yielding absorbance as a function of the dye exclusion method. This has been applied in measurement of interleukin-2 activity in a multiwell assay. The concentration used may be reduced by dilution of powder, BioReagent, suitable for cell culture the stock solution (5 mg/ml) to 0. This method is based on the principle that live (viable) cells do not take up certain dyes, whereas dead (non-viable) cells do. If the background is too dark, cells should be pelleted and resuspended in protein-free medium or salt solution prior to counting. Carefully touch the edge of the cover-slip with the pipette tip and allow each chamber to fill by capillary action. Do not the circle indicates the approximate area covered at 100x microscope overfill or underfill the chambers. Do not count cells touching middle line at bottom 4) Starting with chamber 1 of the hemacytometer, count all the cells in the 1 and right. Count 4 corner squares and middle square in both chambers mm center square and four 1 mm corner squares (see Diagram I). Some cells may detach more quickly than others as a function of where they are in the cell cycle and the amount of extracellular matrix present. Add an equal volume (or a minimum of 5 ml) of Trypsin Inhibitor solution (T0800) to the cell suspension and transfer to a sterile centrifuge tube. The importance of cytokines and growth factors in cell culture has been endotoxin. Serum and other undefined extracts and the painstaking, continued attempts to characterize them have greatly contributed to this situation. Directions for Thawing Human Endothelial Cells Studies published as early as 1921 acknowledged the fact that there were 1. Aspirate supernatant, leaving a even when all the necessary nutrients are present certain cells especially minimal volume to cover cells. Add Endothelial Cell Growth Factor (E9640) to loosened cell pellet at 1% of factors that encouraged cell proliferation. Plate cells on Endothelial Cell Attachment Factor (E9765) coated tissue Growth factors can be described as proteins that bind to receptors on the culture surface. Cytokines are generally thought of as part of the Amphiregulin human signaling mechanism that orchestrates the immune response to bacterial infection. In both the research and pharmaceutical community, there is a growing Lyophilized from 0. This is always true for primary cell lines but often necessary for transformed cell lines and mol wt ~11 kDa hybridomas as well. Selecting the appropriate growth factors and cytokines for your application is endotoxin. Within this section, you will find s listing of cell culture tested cytokines and growth factors along with helpful information that will make Betacellulin human choosing the products you need easier. Inhibits the growth of several epithelial tumor cells and stimulates the When ErbB-2 is combined into a heterodimer with ErbB-3 or ErbB-4, the growth of fibroblasts and other cell types. Its expression is upregulated in a binding affinities of both and isoforms are substantially improved. It is effective in a prinicple mechanism of their release into extracellular fluid has not yet been variety of fibroblast and endothelial cell types and also acts synergistically resolved. They are chemotactic and mitogenic for endothelial cells and induce the media grade release of agents that break down basement membranes. L6 myoblasts They also appear to participate in some pathological conditions that involve excessive cell proliferation or angiogenesis, such as tumor production. Please check with your local supplier regarding availability and restrictions in other countries. The biological activity is measured by the dose-dependent stimulation of Lyophilized from a 0. A natural mitogenic growth factor used in cell culture applications to study endotoxin. The proliferative activity is tested in culture using fetal bovine heart A natural mitogenic growth factor used in cell culture applications to study endothelial cells. Expression of Flt-3 is primarily restricted the biological activity is measured in a cell proliferation assay using a murine among hematopoietic cells to the most primitive progenitor cells. Glycosylation contributes to stability in cell growth media and neutrophils and mature granulocytes, and promotes differentiation of certain other applications. The soluble is gene sequence for the entire cytoplasmic and transmembrane domains and thought to exist in solution as a noncovalently linked dimer. Stem Cells 16, 11-19 suitable for cell culture (1998) the hepatocyte growth factor gene spans ~70 kb and consists of 18 exons 3. Glycosylation contributes to stability in cell growth media and other Erythropoietin is a glycoprotein that is the principal regulator of red blood applications. The specific activity was determined by the dose-dependent stimulation of endotoxin. Glycosylation contributes dimer mol wt 16 kDa (glycosylated) to stability in cell growth media and other applications. Glycosylation contributes to ship: ambient store at: room temp stability in cell growth media and other applications. Production in human 293 cells monomer with an apparent molecular mass of 17-45 kDa due to offers authentic glycosylation. Glycosylation contributes to stability in cell glycosylation, which is absent when this cytokine is expressed in E. Glycosylation contributes to stability molecular masses of 35, 40, and 45 kDa, respectively. Oncostatin M is a growth-regulating cytokine, affecting a number of tumor and normal cells. Glycosylation contributes to stability in cell growth liver and acts as an endocrine to distant cells. Glycosylation contributes to stability in cell apoptosis treatments inhibit apoptotic pathways without actually preventing growth media and other applications. Glycosylation contributes Insulin-like Growth Factor-I human to stability in cell growth media and other applications. Interleukin-1 human Recombinant analog of insulin-like growth factor-I with the substitution of Arg for Glu at position 3. Interleukin-2 plays a role in the activation and stimulate cell proliferation using a mouse helper T cell line, D10. This lymphokine promotes long term growth of activated T secretion from activated B cells. The biological activity is measured by the dose-dependent stimulation of the endotoxin. It acts upon a variety of cells, including fibroblasts, myeloid progenitor cells, T cells, B cells and hepatocytes. Human and rat ciliary neurotrophic factor share ~83% neurons in the substantia nigra, basal forebrain cholinergic neurons, sequence homology and show cross-reactivity in bioactivity. The proliferative activity is tested in a cell proliferation assay using the the product is lyophilized from a sterile 0. It has identical amino acid sequence in human, mouse, mitogenic for dermal and tendon fibroblasts, vascular smooth muscle cells, and pig with full cross-reactivities. It is critical for proprioceptive be pathogenic in arteriosclerosis and neoplasia. It is mitogenic to recombinant, expressed in Escherichia coli, lyophilized powder, mesoderm-derived cells, such as dermal and tendon fibroblasts, vascular suitable for cell culture smooth muscle cells, glial cells, and chondrocytes. Glycosylation contributes to stability in cell growth media and other applications. The specific activity was determined by the dose-dependent stimulation of the proliferation of 3T3 cells. Activins influence erythropoiesis and the potentia reversibly confers a transformed phenotype upon normal non-neoplastic tion of erythroid colony formation, oxytocin secretion, paracrine, and cells, such as normal rat kidney fibroblasts. One unit is the amount required to induce half-maximal cytolysis of L929 mol wt ~17. During embryo development it is expressed in the cephalic secreted homodimeric, heparin-binding glycoprotein,1 which has an iso mesenchyme, tail region and allantois and along the somites. Estradiol E1024-1G E1024-25G E1024-100G -Estradiol To prepare a 20 g/ml stock solution, add 1 ml absolute potency 0. E2758-5G -Estradiol To prepare 20 g/ml stock solution: add 1 ml absolute potency 0. E2758-1G 1g An anti-inflammatory glucocorticoid with a range of effects on cell survival, E2758-5G 5g cell signaling and gene expression. Add 49 ml sterile medium per ml of ethanol added, while mixing, to acheive final concentration of 20 g/ml. To prepare 20 g/ml stock solution: add 1 ml absolute ethanol; gently swirl to Package size based on estradiol dissolve; add 49 ml sterile medium while mixing. It has three times the anti-inflammatory potency of corticosterone but much lower Na2+ retention cell signaling and gene expression. Package size based on dexamethasone -irradiated, powder, BioXtra, suitable for cell culture solubility Hydrocortisone for use in epithelial and endothelial adherent cell culture H O. Estrogens direct 98% the development of the female phenotype in embryogenesis and during To prepare 50 g/mL stock solution, add 1.
Buy altace 1.25 mg lowest price. How to take blood pressure - Clinical skills for student nurses.